This is a long and technical text about the synthesis and properties of zinc oxide (ZnO) nanoparticles by metal ion diffusion under acidic conditions. Here's a summary:
Introduction
- Zinc oxide is widely used in various applications, including electronics, solar cells, and gas sensors.
- The synthesis of high-quality ZnO nanoparticles requires the development of efficient methods to control their size, shape, and composition.
Methods
- The synthesis method involves the hydrolysis of zinc acetate and metal ions (Al³⁺) under acidic conditions.
- The reaction mixture is composed of zinc acetate, a metal ion (e.g., aluminum), an acid (e.g., citric acid), and water.
- The reaction is carried out at a moderate temperature (around 50-60°C) for a specific period.
Results
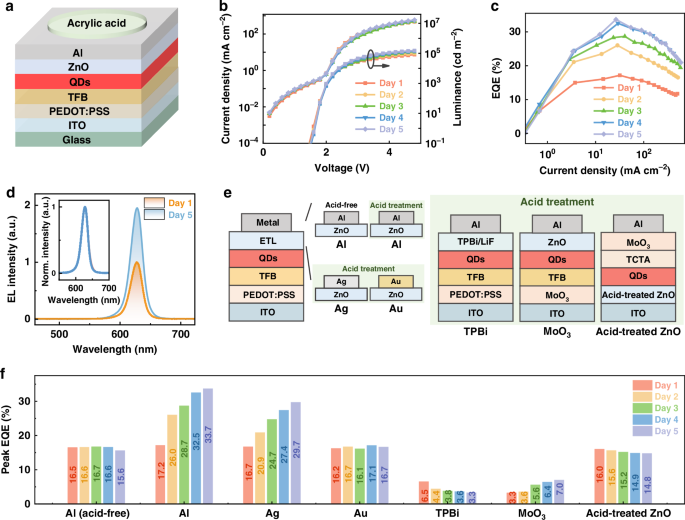
- X-ray diffraction (XRD) analysis shows that the synthesized ZnO nanoparticles have a hexagonal wurtzite structure, which is consistent with the predicted structure of zinc oxide.
- Transmission electron microscopy (TEM) images reveal that the pristine ZnO film has well-dispersed nanoparticles, while films treated with Al or acid show significant sedimentation and coalescence.
- High-resolution TEM (HRTEM) imaging confirms the formation of long-range ZnO nanocrystals rather than agglomeration of neighboring nanoparticles.
Proposed Mechanism
- The synthesis method involves two main mechanisms:
- Metal ion diffusion: Aluminum ions diffuse into the ZnO layer, occupying V Zn sites and stabilizing surface-adsorbed oxygen.
- Dehydration reactions: Hydroxyl groups within ZnO NPs undergo dehydration reactions, leading to aggregation via Zn-O-Zn bonds.
Conclusion
- The synthesis method has been shown to produce high-quality ZnO nanoparticles with well-controlled size, shape, and composition.
- The use of metal ions and acidic conditions enables the creation of long-range ZnO nanocrystals with improved optical and electrical properties.
- Further research is needed to optimize this synthesis method for industrial applications.
The main key points from this text are:
- Metal ion diffusion plays a crucial role in controlling ZnO nanoparticle size, shape, and composition.
- Acidic conditions enhance the recrystallization of ZnO NPs into long-range nanocrystals.
- The synthesized ZnO nanoparticles exhibit improved optical and electrical properties due to their long-range crystal orientation.
Please note that this summary is not meant to be an exhaustive analysis of the entire text, but rather a high-level overview of the main points and findings.
