India to Have Reliable and Affordable Medical Assistive Technology through New Standards
New Delhi, Dec 2023: The Bureau of Indian Standards (BIS), India's national standards body, is addressing the growing need for reliable and affordable medical assistive technology. In line with the National Medical Device Policy, 2023, BIS will prioritize standards development for 214 critical medical devices by December 2025.
These critical devices include septal closure devices, plasma sterilizers, and phototherapy machines. The initiative is set to be phased completed by December 2025, with consultations made with the Department of Pharmaceuticals (DoP) in identifying these essential medical devices.
BIS has taken a significant step towards driving improvements in healthcare quality, safety, and reliability by developing robust medical device and service standards. By aligning with the Medical Devices Rules, 2017, and the National Medical Device Policy, 2023, BIS is establishing a robust regulatory framework that prioritizes public safety while fostering innovation.
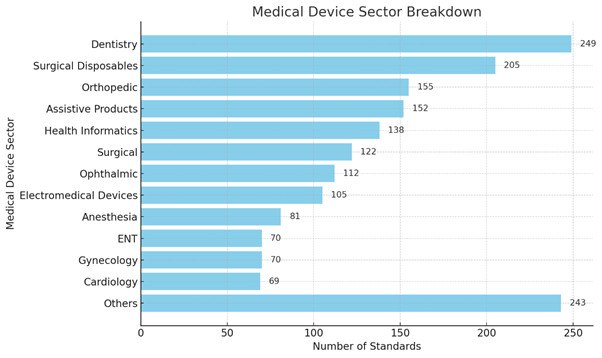
The Bureau has published over 1,700 standards for the medical sector, covering specialties such as cardiology, neurology, orthopaedics, ophthalmology, among others. Of these, around 1,200 standards focus on medical devices critical to healthcare, including life-saving devices like cardiac pacemakers and heart valves, advanced diagnostic tools such as X-ray machines and MRI systems, with the inclusion of assistive technology devices.
For instance: India's Heart Valve standard (Isapac 2021), hearing aids, wheelchairs, Jaipur Foot, tactile pathways for visually impaired individuals are some examples. These standards ensure that Indian medical devices are safe, effective, and globally competitive, solidifying India's reputation as a leader in healthcare innovation.
The inclusion of assistive technologies aims to bridge the gap between healthcare accessibility and affordability, catering to the needs of individuals with disabilities. BIS will continue to drive this effort towards improved quality of life for patients through reliable medical devices that cater to diverse requirements, thereby promoting healthy living and growth in India's healthcare sector.
These latest standards development initiatives reflect the growing commitment of BIS towards advancing medical technology regulations and ensuring greater public safety while catering to a rapidly evolving demand for modern healthcare solutions.
Disclaimer: News Published by Alarian.com
