Global Health Breakthrough: WHO Recommends Maternal Vaccination and Antibodies to Prevent Deadly RSV Disease
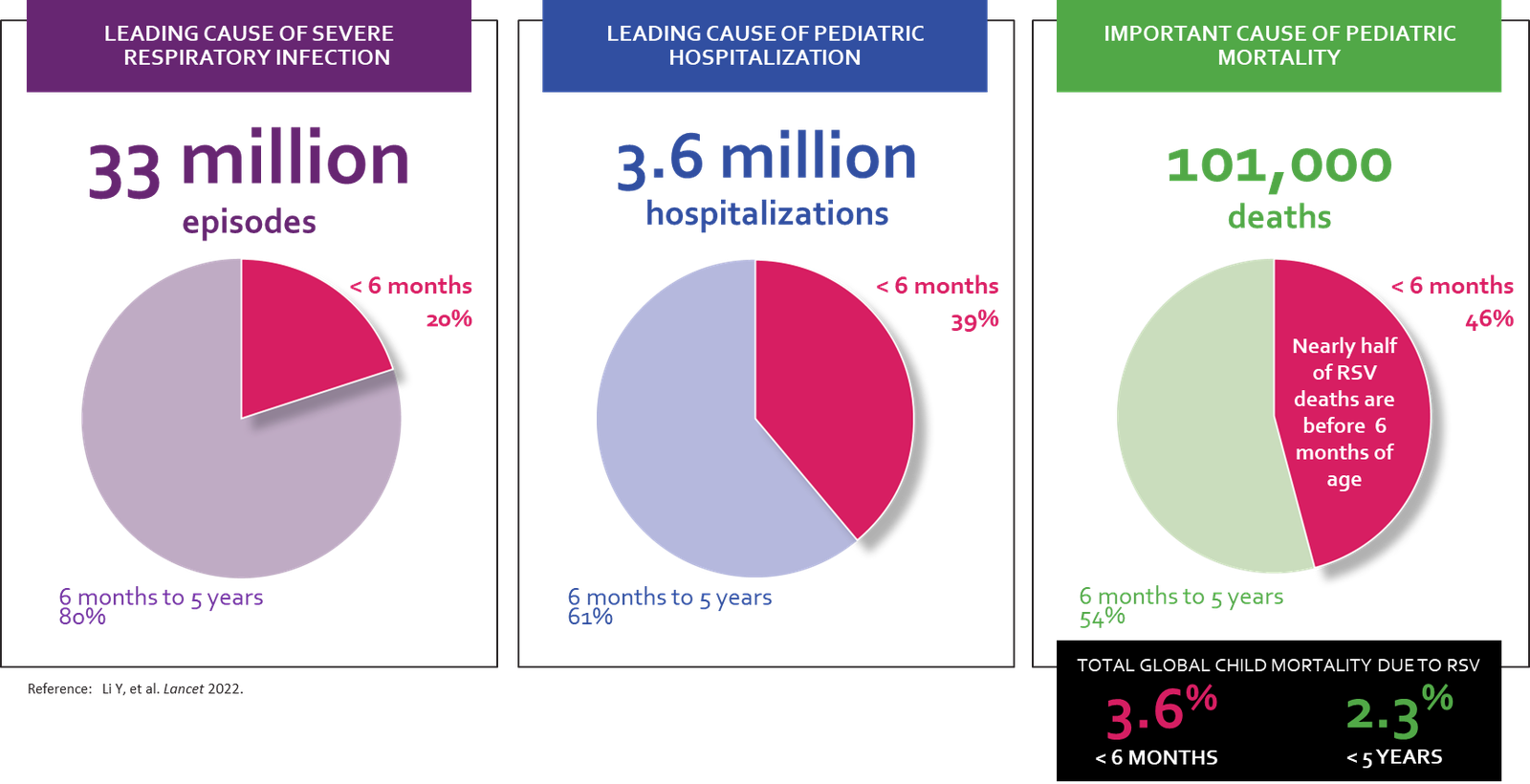
GENEVA, SEPTEMBER 10, 2024 - In a significant milestone, the World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) has recommended that all countries adopt maternal vaccination and/or long-acting monoclonal antibodies to prevent severe respiratory syncytial virus (RSV) disease in young infants. This welcome news marks an exciting step towards global scale-up and use of these life-saving interventions.
However, the task now lies in raising awareness about the benefits of these new therapies, overcoming barriers to introduction, and accelerating the product availability and use in low- and middle-income markets where need is greatest. The WHO's announcement highlights the urgent need for increased access to RSV-prevention measures, especially among vulnerable populations such as infants in developing countries.
To address these challenges, international partners, including PATH, have been working diligently to develop tools and evidence-based resources to support informed decision-making and implementation planning around RSV and its interventions. These efforts aim to ensure that these groundbreaking treatments reach those who need them most, saving countless lives worldwide.
As the global healthcare community continues to progress on this critical front, all eyes are now fixed on accelerating the pace of innovation, collaboration, and action towards making RSV-prevention a reality for every infant in need.
